How to Master the Validation Lifecycle: A Practical Guide for Quality Engineers
Did you know that for companies implementing cloud-based quality management systems, the validation burden is automatically reduced by roughly 75% compared to on-premise solutions? The validation lifecycle has become a critical foundation for quality engineers working in regulated environments today. Software validation is a mandated requirement in industries such as pharmaceutical, medical device, and food manufacturing, where quality isn’t just a goal—it’s a regulatory obligation.
In the world of quality engineering, we cannot ensure product excellence by testing only the finished product. Instead, we must build quality, efficiency, and safety into each step of the manufacturing process. This principle underlies the validation lifecycle approach, which has been formalized through guidance published by the FDA in 2011 and later by PIC/S and EMA in 2015. The pharmaceutical industry, for example, is currently undergoing a significant transformation as it embraces this revised guidance, introducing the validation lifecycle approach.
In this practical guide, we’ll walk through the essential components of validation lifecycle management systems and explore how quality engineers can master the process validation lifecycle. From understanding the five general steps of software validation (requirement management, project planning, testing, reporting, and maintenance) to distinguishing between commissioning and qualification documentation, we’ll provide you with actionable insights to enhance your validation practices. By the end of this article, you’ll have a comprehensive understanding of how to implement and optimize validation lifecycle processes within your organization.
Understanding the Validation Lifecycle in Quality Engineering
The validation lifecycle encompasses a systematic series of critical processes designed to ensure systems, equipment, and processes consistently perform their intended functions effectively. Process validation is formally defined as “the collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality product”. This comprehensive approach has evolved from a one-time validation event to a continuous lifecycle concept with three distinct stages.
Definition of validation lifecycle and its scope
The validation lifecycle incorporates a continuous feedback loop during system, equipment, and software validation. It spans from initial design through implementation and ongoing verification, ensuring that assessments, deliverables, and testing adhere to predetermined specifications and quality attributes throughout a product’s existence. This lifecycle approach includes : Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). The FDA’s guidance document from 2011 formalized this approach into three stages: Process Design, Process Qualification, and Continued Process Verification.three primary phases
Why validation matters in regulated and non-regulated industries
In regulated industries like pharmaceuticals and medical devices, validation is a mandated practice integral to the entire drug development process. Regulatory bodies including the FDA, EMA, and others require documented evidence of process validation as part of compliance frameworks. Nevertheless, non-regulated industries also benefit from implementing validation practices:
- Risk mitigation and enhanced product quality
- Improved operational efficiency and streamlined processes
- Increased customer confidence and competitive advantage
- Enhanced data integrity and decision-making capabilities
How validation lifecycle supports compliance and quality assurance
The validation lifecycle strengthens compliance by verifying that assessments, deliverables, and implementation adhere to validation requirements while systems remain in use. Consequently, organizations can enforce standardization, optimize processes, achieve data integrity, reduce risks, and maintain regulatory compliance. Furthermore, validation lifecycle management assists in preparing for regulatory audits by providing a system of record for evidence. Quality management systems benefit directly as validation provides documented evidence that processes operate consistently within established parameters, helping identify critical quality attributes and creating the foundation for continuous improvement initiatives.
Breaking Down the Validation Lifecycle Model
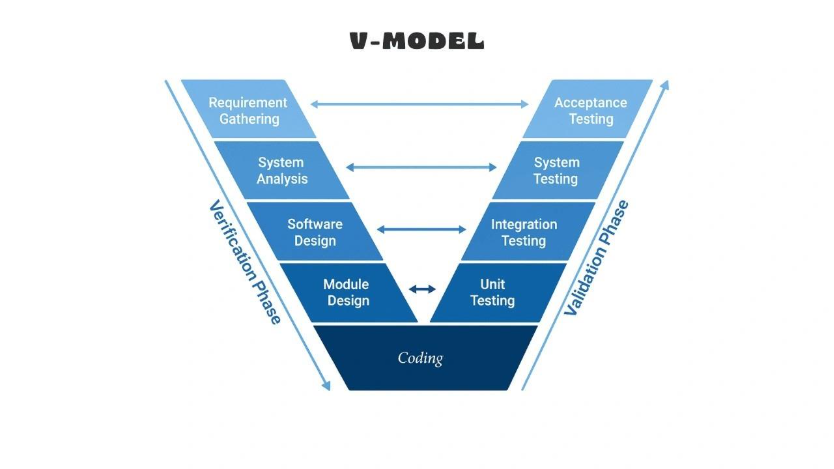
Image Source: Teaching Agile
The validation lifecycle model consists of five interconnected stages that form the backbone of effective quality assurance in regulated environments. Each phase builds upon the previous one, creating a comprehensive approach to ensure systems consistently meet requirements.
Requirements Gathering and Risk Assessment
Effective validation begins with thorough requirements gathering, which involves identifying stakeholders and documenting their expectations. Quality engineers must collect detailed information about processes, systems, and equipment through structured interviews and documentation. Specifically, a well-executed requirements gathering process improves stakeholder satisfaction, increases project success rates, and reduces costs. According to NASA studies, projects that spent less than 5% of total costs on requirements processes experienced 80-200% cost overruns, whereas those investing 8-14% experienced less than 60% overrun
Risk assessment subsequently guides validation priorities by identifying potential failure modes. This critical step employs a systematic methodology to evaluate the likelihood and severity of risks, particularly focusing on patient safety, data integrity, and regulatory compliance. Quality teams should develop clear scales for probability and impact to ensure consistent risk evaluation.
Validation Planning and Protocol Development
A validation protocol serves as the core document describing objectives, design, methodology, statistical considerations, and organization of the validation effort. Protocols must adhere to regulatory standards like the SPIRIT statement for clinical trials, ensuring all essential scientific and administrative elements are addressed. The protocol starts by providing background information, then proceeds to outline the validation plan with carefully designed test scripts.
Test Execution and Traceability Matrix
During test execution, predefined cases are run in real-world or simulated environments to verify . This phase is pivotal in pharmaceutical and biotech industries due to the high stakes involved—patient safety, regulatory approval, and operational integrity. The traceability matrix establishes critical links between requirements and test cases, ensuring complete verification coverage. This document enables bidirectional tracing, allowing teams to track both forward (to implementation) and backward (to requirements origin).system functionality and compliance
Validation Reporting and Summary Documentation
A Validation Summary Report (VSR) provides a concise overview of the entire validation effort and results obtained. This document should address: system identification, inventory of deliverables, summary of test results, deviations from the plan, outstanding issues, and a statement of acceptance. The VSR serves as authorization for system release into operational use, essentially demonstrating that validation activities were performed according to approved procedures.
Ongoing Maintenance and Change Control
Finally, maintaining validated status requires ongoing vigilance. Change control demonstrates to regulatory authorities that validated systems remain controlled throughout their lifecycle. Organizations must define explicit processes for evaluating changes, with a multidisciplinary approach to considering potential effects. According to industry standards, changes typically fall into three categories: minor (requiring limited testing), major (needing additional re-validation), and critical (triggering complete re-validation). Regular monitoring ensures systems remain in a state of control through requalification/revalidation.
Common Challenges and How to Overcome Them
Quality engineers face numerous obstacles throughout the validation lifecycle. Understanding these challenges and implementing effective strategies is crucial for maintaining compliant, efficient validation processes.
Documentation overload and traceability gaps
Software validation produces substantial documentation, often leading to information overload. Studies indicate that is consumed by rework activities, frequently resulting from traceability gaps. Moreover, organizations struggle with missing links between User Requirements Specification (URS), design documents, and test scripts. These gaps create inconsistent documentation of IQ, OQ, and PQ results, making compliance demonstration difficult during inspections.between 40% and 70% of a project’s budget
To address these issues, implement risk-based validation approaches that prioritize critical systems. Maintain complete documentation ensuring all requirements, test scripts, results, and deviations are documented and traceable. Additionally, establish a digital thread across the entire V-model to enable live traceability.
Audit readiness and regulatory expectations
Lack of audit readiness often leads to costly delays and diminished stakeholder confidence. Common audit failures stem from incomplete traceability documentation, particularly when validating integrated processes or software platforms. Unprepared companies typically experience higher-than-expected fees, identification of internal control deficiencies, and potentially irreparable harm to financing capabilities.
Proper preparation includes documenting internal control processes comprehensively and conducting periodic reviews to identify potential gaps. Furthermore, strengthen data integrity controls through enforced user access protocols and secure electronic records.
Managing validation in cloud-based vs on-premise systems
On-premise systems require internal teams to validate the software environment and maintain change control, whereas cloud-based platforms often come pre-validated. Despite advantages, cloud systems present unique challenges since updates can occur unbeknownst to users.
The validation relationship between cloud software vendors and clients differs fundamentally from on-premises solutions. Review Master Service Agreements (MSAs) that clearly outline uptime, backup practices, and security measures. Utilize third-party certifications like System and Organization Controls (SOC) compliance, and thoroughly evaluate the vendor’s validation program.
Handling frequent software updates and revalidation cycles
FDA analysis reveals that were caused by defects introduced after initial production. Unfortunately, traditional expectations of revalidating software following each update present significant challenges, particularly with frequent Windows patches and updates.79% of software-related recalls
Implement a risk-based approach to revalidation, focusing only on high-risk changes. Create a change control approach that allows quick determination of necessary system changes. Additionally, consider upgrading frequently rather than postponing updates, as this actually reduces validation burden over time—small, frequent changes require less validation effort than major upgrades after extended delays.
Tools and Technologies for Validation Lifecycle Management
Modern technology has revolutionized validation lifecycle management, with specialized tools creating significant efficiency improvements. Effective digital solutions now support every stage of the validation process, offering structured frameworks that ensure consistent compliance.
Validation lifecycle management system overview
Validation Lifecycle Management Systems (VLMS) provide centralized platforms for managing validation activities across the entire lifecycle. These systems maintain inventories of validation items, standardize requirements management, and automatically close change actions. They establish a “single source of truth,” enabling cross-functional collaboration and standardized workflows that minimize errors. Indeed, modern VLMS platforms incorporate risk assessment tools alongside mechanisms for audit preparation.
Automated test execution and regression tools
Regression testing tools automate tedious validation tasks, allowing teams to focus on software development while ensuring quality. These tools detect visual changes between application versions and support web, mobile, and API testing. Importantly, they execute tests faster through parallel testing, which significantly accelerates feedback cycles. Overall, automated test execution reduces the most time-consuming validation phase—testing—while generating structured, audit-ready documentation.
Digital templates for URS, OQ, PQ, and traceability
Digital templates standardize validation documentation, reducing authoring time by up to 70%. Pre-built User Requirements Specifications (URS) templates include sections for user characteristics, data requirements, functionality needs, interface requirements, and compliance expectations. Similarly, OQ/PQ templates provide structured formats for test descriptions, data setup, test steps, results review, and tester logs. Alongside these, traceability matrices establish atomic connections between document sections and process elements.
Integration with QMS and document control systems
Although validation is driven by quality events, it often remains disconnected from quality management systems (QMS) in most organizations. This separation creates bottlenecks, as teams manually update multiple systems. Nonetheless, unified validation and QMS workflows can reduce cycle times by 50-70%. Moreover, integration enables teams to link test discrepancies to deviations and trace change impacts without manual reconciliation.
Benefits of cloud-based validation platforms
Cloud-based validation platforms deliver numerous advantages compared to on-premises solutions:
- Automatic disaster recovery, backups, and updates included in subscriptions
- Seamless provisioning of test environments as needed
- Remote access from any location with internet connectivity
- Reduced implementation time through cloud automation tools
- Ability to delegate validation activities to global teams
- Automatic generation of validation reports
Conclusion
Mastering the validation lifecycle represents a critical investment for quality engineers working in today’s complex regulatory environments. Throughout this guide, we’ve explored how the validation process has evolved from a one-time event into a continuous, integrated lifecycle approach encompassing requirements gathering, planning, execution, reporting, and ongoing maintenance.
Above all, effective validation lifecycle management delivers tangible benefits beyond mere regulatory compliance. Organizations that implement structured validation practices experience improved product quality, enhanced operational efficiency, and significantly reduced risk profiles. The systematic approach outlined in this article helps quality engineers establish consistent, repeatable processes that withstand regulatory scrutiny while supporting continuous improvement initiatives.
Undoubtedly, challenges persist in validation management – from documentation overload to managing frequent software updates. However, these obstacles become manageable through risk-based approaches, standardized documentation practices, and implementation of modern validation lifecycle management systems. Quality engineers who adopt these strategies position themselves as valuable assets within their organizations.
Additionally, the technological landscape continues to evolve, offering powerful tools that automate tedious validation tasks. Cloud-based platforms, digital templates, and integrated quality management systems drastically reduce validation burdens while enhancing traceability. These advancements allow quality engineers to focus on higher-value activities rather than administrative documentation.
Looking ahead, quality professionals who master the validation lifecycle will find themselves well-positioned to navigate the increasingly complex intersection of technology, regulation, and quality assurance. The principles discussed here provide a foundation for building robust validation practices that scale with organizational growth and adapt to changing regulatory expectations.
Remember that validation excellence doesn’t happen overnight – it requires deliberate effort, continuous learning, and systematic implementation. Still, the investment pays dividends through streamlined operations, enhanced compliance posture, and ultimately, superior product quality and safety. By embracing the validation lifecycle approach outlined in this guide, quality engineers can transform validation from a regulatory burden into a strategic competitive advantage.
Key Takeaways
Master these essential validation lifecycle principles to transform regulatory compliance from a burden into a strategic advantage for quality engineering excellence.
• Adopt a continuous lifecycle approach: Move beyond one-time validation events to implement the FDA’s three-stage model (Process Design, Process Qualification, Continued Process Verification) for sustained compliance.
• Implement risk-based validation strategies: Prioritize critical systems and focus validation efforts where they matter most—studies show proper requirements gathering reduces cost overruns from 200% to under 60%.
• Leverage digital tools for efficiency: Modern validation lifecycle management systems reduce documentation time by 70% and cycle times by 50-70% through automation and standardized templates.
• Establish robust traceability matrices: Create bidirectional links between requirements and test cases to prevent the 40-70% project budget waste typically caused by rework from traceability gaps.
• Embrace cloud-based platforms strategically: Cloud solutions reduce validation burden by 75% compared to on-premise systems while providing automatic updates, disaster recovery, and global accessibility.
The validation lifecycle isn’t just about meeting regulatory requirements—it’s about building quality into every step of your processes. Organizations that master these principles experience improved product quality, enhanced operational efficiency, and significantly reduced risk profiles while maintaining audit readiness.
FAQs
Q1. What are the key stages of the validation lifecycle? The validation lifecycle typically consists of five main stages: requirements gathering and risk assessment, validation planning and protocol development, test execution and traceability matrix creation, validation reporting and summary documentation, and ongoing maintenance and change control.
Q2. How does a Validation Master Plan (VMP) contribute to the validation process? A Validation Master Plan outlines the principles for qualifying a facility, defines areas and systems to be validated, and provides a written program for achieving and maintaining a qualified drug manufacturing facility. It serves as a roadmap for the entire validation process.
Q3. What are the primary responsibilities of a quality validation engineer? A quality validation engineer ensures overall product quality, compliance with regulations, and oversees validation activities such as equipment qualification and process validation. They also manage quality systems, conduct audits, control documentation, and ensure GMP compliance.
Q4. How can organizations overcome documentation overload in validation? To address documentation overload, organizations can implement risk-based validation approaches, maintain complete and traceable documentation, and utilize digital tools like validation lifecycle management systems. These strategies can significantly reduce documentation time and improve efficiency.
Q5. What are the advantages of cloud-based validation platforms? Cloud-based validation platforms offer several benefits, including automatic updates and disaster recovery, seamless provisioning of test environments, remote access capabilities, reduced implementation time, and the ability to delegate validation activities globally. They can also automatically generate validation reports, streamlining the entire process.
Recent Post
- Artificial Intelligence Compliance Monitoring in Life Sciences: A New Standard for GxP Excellence
- Mastering Computer System Validation: A Practical Guide for Life Sciences
- What is the Importance of the Validation of the System: A Pillar of Life Sciences
- The V-Model in Computer System Validation (CSV): A Strategic Framework for Life Sciences Compliance
- GCP Audit (Good Clinical Practice Audit): Ensuring Clinical Trial Compliance and Quality
