In today’s digital-first life sciences environment, pharmaceutical, biotech, and medical device companies face relentless regulatory scrutiny. Agencies like the FDA and EMA require firms to demonstrate continuous control of their Good Practice (GxP) processes to ensure product quality, data integrity, and patient safety. Current Good Manufacturing Practice (CGMP) regulations set minimum standards for manufacturing methods
Read MoreIn today’s highly regulated life sciences landscape—encompassing pharmaceuticals, biotechnology, and medical devices—the reliability of computer systems is not merely a technical concern but a fundamental patient safety imperative. Computer System Validation (CSV) serves as the critical bridge between advanced technology and stringent regulatory compliance, ensuring that every system performs its intended function consistently and reliably.
Read MoreIn the high-stakes world of pharmaceuticals, biotechnology, and medical devices, the importance of the validation of the system cannot be overstated. It is the fundamental, non-negotiable process that stands between a functional software application and a trustworthy, compliant asset that protects patient lives and ensures product quality. Understanding the importance of the validation of the
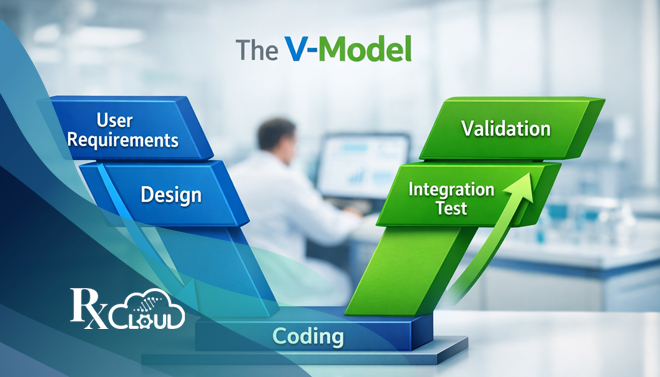
Read MoreIn the highly regulated landscape of life sciences—where a single software flaw can impact patient safety and regulatory approval—the validation of computer systems is not just best practice; it’s an absolute mandate. For pharmaceutical, biotechnology, and medical device companies, navigating the stringent requirements of Good Practice (GxP) regulations demands a robust, structured, and defensible methodology.
Read MoreIntroduction to GCP Audit In the evolving and highly regulated clinical research environment, maintaining compliance with Good Clinical Practice (GCP) is essential. A GCP Audit (Good Clinical Practice Audit) is a structured and independent evaluation of clinical trial activities to ensure that studies are conducted ethically, safely, and in accordance with regulatory requirements. Clinical trials
Read MoreGVP modules form the cornerstone of modern pharmaceutical safety monitoring in the European Union. According to the World Health Organization (WHO), pharmacovigilance is defined as “The science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem”. We recognize how crucial these practices are for ensuring that
Read MoreGMP auditing serves as the cornerstone of pharmaceutical quality assurance and product safety. As we approach 2025, we’re seeing a growing emphasis on comprehensive audit processes that provide a big-picture look at organizational compliance with Good Manufacturing Practice regulations. These audits typically take for simple assessments and up to ten days for complex manufacturing sites.
Read MoreWhat is Good Pharmacovigilance Practice (GVP)? Good Pharmacovigilance Practice (GVP) is a set of measures drawn up to facilitate the performance of pharmacovigilance in the European Union (EU). It represents the minimum standard for monitoring the safety of medicines available to the public. These practices consist of specific guidelines that help track and report adverse
Read MoreDid you know that for companies implementing cloud-based quality management systems, the validation burden is automatically reduced by roughly 75% compared to on-premise solutions? The validation lifecycle has become a critical foundation for quality engineers working in regulated environments today. Software validation is a mandated requirement in industries such as pharmaceutical, medical device, and food
Read MoreQuality control in pharmacovigilance serves as the foundation for detecting and preventing adverse drug reactions in pharmaceutical products. A pharmacovigilance system is defined as a system used by organizations to fulfill legal requirements related to monitoring the safety of medicinal products and detecting any changes to their benefit-risk balance. Without robust quality control measures, these critical
Read More