The V-Model in Computer System Validation (CSV): A Strategic Framework for Life Sciences Compliance
In the highly regulated landscape of life sciences—where a single software flaw can impact patient safety and regulatory approval—the validation of computer systems is not just best practice; it’s an absolute mandate. For pharmaceutical, biotechnology, and medical device companies, navigating the stringent requirements of Good Practice (GxP) regulations demands a robust, structured, and defensible methodology. This is where the V-Model proves indispensable. Far more than a simple testing strategy, the V-Model in Computer System Validation (CSV) provides a comprehensive, risk-based framework that aligns every phase of development with its corresponding verification activity, ensuring software is built correctly and functions as intended in its regulated environment.
Understanding the Core: What Are CSV and the V-Model?
Computer System Validation (CSV) is the formal, documented process of providing a high degree of assurance that a computer system will consistently operate in accordance with its pre-defined specifications and quality attributes. In sectors regulated by bodies like the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency), CSV is a critical component of quality assurance, directly supporting product safety, efficacy, and data integrity.
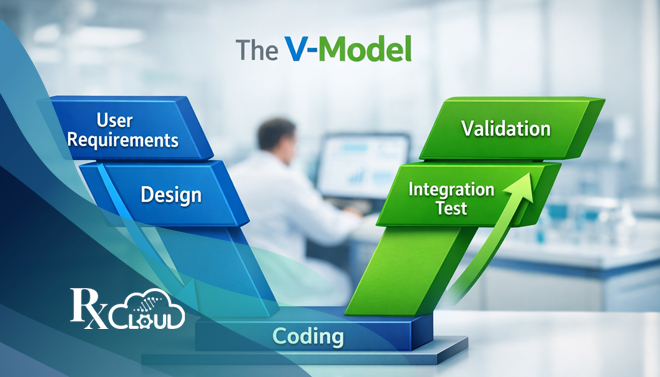
The V-Model is a sequential development lifecycle model that visualizes the relationship between each phase of specification development and its associated testing phase. Its name comes from its characteristic V shape:
- The left descending branch represents the stages of decomposition of user needs into detailed system specifications.
- The right ascending branch represents the stages of integration, testing, and validation, moving from components to the complete system.
- The base of the “V” connects the final, lowest-level design to the most granular unit testing.
Why the V-Model is the Gold Standard for Regulated CSV
For quality and compliance leaders in life sciences, the V-Model offers distinct, critical advantages over less structured approaches. According to services providers like RxCloud, a full-scale quality assurance organization for the life sciences industry, a structured validation framework is key to “streamlining processes, mitigating risks, and upholding the highest quality standards.”
The model’s primary strengths include:
- Inherent Alignment with Regulatory Expectations: Regulatory guidances, such as the FDA’s General Principles of Software Validation, emphasize a risk-based, lifecycle approach. The V-Model provides a clear, auditable trail demonstrating that testing is directly traced to specific requirements—a core regulatory demand.
- Defect Prevention and Early Detection: By forcing detailed specification upfront, errors and ambiguities are caught in the design phase, which is exponentially less costly to fix than defects discovered during system testing or, worse, after go-live.
- Clear Traceability Matrix Foundation: Every test case on the right side of the “V” can be linked directly back to a requirement or specification on the left side. This creates a bullet-proof audit trail, proving that all requirements were tested and all tests were derived from requirements.
- Structured Risk Management: The model facilitates risk assessment at each stage. Critical functions identified in the User Requirements Specification (URS) can be traced through design and subjected to more rigorous testing.
- Efficiency and Predictability: While requiring upfront investment in planning, it reduces rework, prevents project drift, and provides clear milestones and deliverables, ultimately accelerating time-to-market for compliant systems.
Navigating the V-Model: A Stage-by-Stage Guide for CSV
Implementing the V-Model in a CSV project involves specific, documented deliverables at each phase. Here’s a practical walkthrough:
The Left Side of the V: Specification & Design
- User Requirements Specification (URS): This is the foundation. It defines what the system must do from the business and user perspective, often in GxP terms (e.g., “The system must ensure 21 CFR Part 11 compliance for electronic signatures”).
- Functional Specification (FS): This details how the system will meet the URS, describing the software’s functionalities, often without detailing the technical architecture.
- Design Specification (DS): This is the technical blueprint. It describes the system’s architecture, hardware, software, interfaces, and data flows. For configurable Commercial Off-The-Shelf (COTS) products like Veeva or Salesforce, this includes the configuration specifications.
The Right Side of the V: Testing & Validation
- Unit/Module Testing: Corresponding to the DS, developers test individual software units or configuration components to ensure they are built correctly.
- Integration Testing: Corresponding to the FS, this verifies that individually tested modules interact with each other and with external systems as specified.
- User Acceptance Testing (UAT) / System Testing: This critical phase directly tests against the URS. End-users confirm the system performs as required in a simulated or real-life environment. This includes testing for GxP critical processes.
- Operational Qualification (OQ) & Performance Qualification (PQ): Often part of the testing hierarchy, these ensure the system operates consistently under normal and anticipated stress conditions.
Closing the V: Deployment and Ongoing Lifecycle
- The Requirement Traceability Matrix (RTM) is the living document that ties every test on the right back to a specification on the left.
- Final Validation Report summarizes all activities, confirms acceptance, and releases the system for production use.
- The model extends into the operational phase, requiring change management, periodic review, and re-validation as needed—a process supported by ongoing Quality Management System (QMS) consulting and Audit as a Service offerings.
Real-World Application and Common Challenges
A leading pharmaceutical company might employ the V-Model to validate a new Argus Safety system for pharmacovigilance. The URS would capture global safety reporting rules, the FS would detail case processing workflows, and the DS would specify the system configuration. Testing would then validate that individual case forms work (Unit), data flows correctly to regulatory reports (Integration), and the entire system meets global safety compliance needs (UAT).
Common challenges include:
- Inadequate Initial Requirements: Vague or incomplete URS leads to validation gaps. Solution: Invest significant time with cross-functional stakeholders, including Quality and Compliance teams, in the requirements phase.
- Treating CSV as a One-Time Project: Validation is a lifecycle. Solution: Integrate CSV into the organization’s QMS, using change control and periodic review to maintain a validated state.
- Resource and Expertise Constraints: Many organizations lack in-house CSV specialists. Solution: Partner with specialized quality engineering service providers. As highlighted by industry leaders, partners can help “reduce testing cycle times” and bring “over 1000 years of combined IT expertise and in-depth knowledge of quality and compliance.”
Conclusion: Building a Culture of Assured Quality
For life sciences companies, the goal is not merely to pass an audit but to embed quality and compliance into the fabric of their operations. The V-Model in CSV is more than a process diagram; it is a manifestation of a quality-focused culture. It provides the structured, transparent, and traceable methodology needed to ensure that mission-critical computer systems are reliable, compliant, and ultimately, protect patient safety and product integrity.
By adopting this disciplined approach—often with the support of specialized partners who offer “uncompromised quality by reducing process and procedural overheads”—organizations can achieve both regulatory excellence and operational agility, turning compliance from a cost center into a strategic advantage that propels innovation.
Read Also – Introduction to Computer System Validation (CSV)
Frequently Asked Questions (FAQ)
Here are detailed answers to common questions about applying the V-Model in CSV projects.
Q1: What is the V-Model in the context of Computer System Validation (CSV)?
- The V-Model is a structured lifecycle model used in CSV to visualize the direct relationship between each phase of system specification (the left side of the “V”) and its corresponding testing phase (the right side of the “V”).
- Its primary goal is to ensure that every single user requirement is systematically tested and verified, providing documented evidence that the system is fit for its intended use and compliant with regulations like FDA 21 CFR Part 11.
- In regulated industries, it is often implemented following the GAMP 5 framework, which provides a risk-based approach to achieving compliant validation.
Q2: Why is the V-Model considered the gold standard for CSV in life sciences?
The V-Model is favored in pharmaceuticals, biotechnology, and medical devices because it directly addresses core regulatory expectations:
- Clear Traceability: It creates an unbroken audit trail from User Requirements (URS) through design to test scripts and results. This traceability is critical during regulatory inspections.
- Defect Prevention: By requiring detailed specifications upfront, issues are caught in the design phase, which is far less costly to fix than defects found after deployment.
- Structured Risk Management: The model facilitates formal risk assessment at each stage. High-risk functions identified in the requirements can be traced and subjected to more rigorous testing.
- Comprehensive Documentation: It ensures the generation of the necessary documentation (URS, FS, DS, test protocols, reports) that regulators require to prove a system is validated.
Q3: What are the key phases and deliverables in a CSV V-Model project?
The process follows the “V” shape, with corresponding phases opposite each other.
Left Side (Specification & Design):
- User Requirements Specification (URS): Defines what the system must do from a business and compliance perspective.
- Functional Specification (FS): Details how the system will meet the URS, describing software functions and operations.
- Design Specification (DS): The technical blueprint describing system architecture, hardware, software, and configurations.
Right Side (Testing & Verification):
- Unit/Integration Testing: Corresponds to the DS and FS, verifying that individual components and their interactions work correctly. This often aligns with Installation Qualification (IQ) and parts of Operational Qualification (OQ).
- System Testing / User Acceptance Testing (UAT): Directly tests against the URS in a simulated environment. This is the core of OQ and Performance Qualification (PQ), proving the system works as required under real-world conditions.
- The central, living document that ties everything together is the Requirement Traceability Matrix (RTM), ensuring no requirement is left untested.
Q4: Isn’t the V-Model too rigid for modern, fast-paced development?
- The traditional V-Model is indeed a sequential and linear approach, which can be challenging if requirements change frequently.
- However, for validating core, stable GxP systems (like ERP, LIMS, or manufacturing execution systems) where requirements are well-understood and patient safety is paramount, its rigidity is a strength that ensures control and compliance.
- For projects requiring more speed and flexibility, organizations can adopt a hybrid “Agile-V” approach. This involves using agile sprints for development while mapping deliverables and tests back to the V-Model framework to maintain validation integrity and documentation.
Q5: How does the V-Model support compliance with GAMP 5 guidelines?
- GAMP 5 is the industry’s risk-based framework for validating computerized systems. The V-Model is the most common lifecycle model used to implement GAMP 5.
- The V-Model’s phases align perfectly with GAMP 5’s core concept of a “lifecycle approach,” which includes planning, specification, verification, and reporting.
- It directly supports the GAMP 5 principle of leveraging supplier involvement and defining software categories (e.g., configured vs. custom software), as the validation strategy on the left side of the “V” is tailored based on this categorization and risk assessment.
Recent Post
- Artificial Intelligence Compliance Monitoring in Life Sciences: A New Standard for GxP Excellence
- Mastering Computer System Validation: A Practical Guide for Life Sciences
- What is the Importance of the Validation of the System: A Pillar of Life Sciences
- The V-Model in Computer System Validation (CSV): A Strategic Framework for Life Sciences Compliance
- GCP Audit (Good Clinical Practice Audit): Ensuring Clinical Trial Compliance and Quality