Our Thought Leadership & Industry Trends
Artificial Intelligence Compliance Monitoring in Life Sciences: A New Standard for GxP Excellence
In today’s digital-first life sciences environment, pharmaceutical, biotech, and medical...
Mastering Computer System Validation: A Practical Guide for Life Sciences
In today’s highly regulated life sciences landscape—encompassing pharmaceuticals, biotechnology, and...
What is the Importance of the Validation of the System: A Pillar of Life Sciences
In the high-stakes world of pharmaceuticals, biotechnology, and medical devices,...
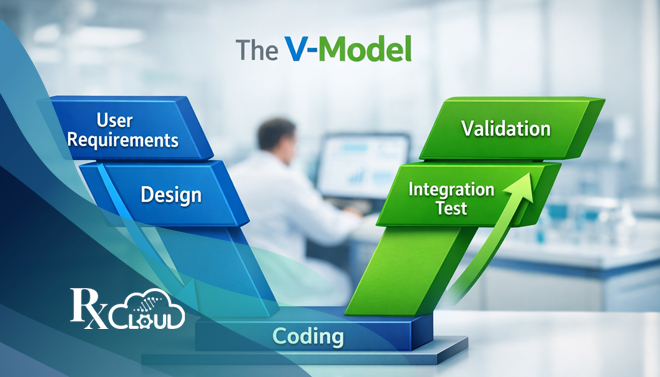
The V-Model in Computer System Validation (CSV): A Strategic Framework for Life Sciences Compliance
In the highly regulated landscape of life sciences—where a single...
GCP Audit (Good Clinical Practice Audit): Ensuring Clinical Trial Compliance and Quality
Introduction to GCP Audit In the evolving and highly regulated...
Good Pharmacovigilance Practice (GVP) Modules: Expert Implementation Guide
GVP modules form the cornerstone of modern pharmaceutical safety monitoring...
GMP Auditing: What Quality Managers Need to Know in 2025
GMP auditing serves as the cornerstone of pharmaceutical quality assurance...
What Is GVP? A Guide to Good Pharmacovigilance Practices
What is Good Pharmacovigilance Practice (GVP)? Good Pharmacovigilance Practice (GVP)...
How to Master the Validation Lifecycle: A Practical Guide for Quality Engineers
Did you know that for companies implementing cloud-based quality management...
Quality Control in Pharmacovigilance: Essential Standards for Risk Prevention
Quality control in pharmacovigilance serves as the foundation for detecting...
Audit Readiness Made Simple: Your Life Sciences Success Blueprint
A regulatory audit is one of the most high-stakes events...
Why Quality Assurance Fails Without Regulatory Compliance: Expert Analysis
Quality assurance and compliance are inseparable partners in today’s manufacturing...